Iec 60601-1 | Iec 60601 Medical Electrical Equipment Classification Faqs Medical Device Academy Medical Device Academy
IEC 60601-12021 SER Standard electromagnetic compatibility EMC smart city Medical electrical equipment - ALL PARTS. The table at the beginning of this blog posting identifies the five parts of the Classification section.

Elexes Blog Iec 60601 1 Evolution Of Electrical Safety
What are the various classifications that are used in IEC 60601-1 edition 31.
Iec 60601-1. Further checking in the standard reveals that Annex F is intended for outdoor noise measurements where reflections are. The original IEC 60601-1 for medical devices was published in 1977. The force of this standard is to require two level of protection to guard the patient and operator from any injury.
IEC 60601-1-112015 applies to the basic safety and essential performance of medical electrical equipment and medical electrical systems for use in the home healthcare environment. It applies regardless of whether the medical electrical equipment or medical electrical system is intended for use by a lay operator or by trained healthcare personnel. MECA provides high-quality testing and documentation necessary to show compliance with medical and laboratory equipment standards primarily related to the IEC 60601-1 and IEC 61010-1 series of standards.
IEC 60601-1-82006 Medical electrical equipment Part 1-8. IEC 60601-1 Edition 31 2012-08 INTERNATIONAL STANDARD Medical electrical equipment Part 1. IEC is an acronym for the International.
Of IEC 60601-1-2 are the same. IEC 60601-1 Compliance Documents to evaluate medical electrical equipment to the applicable standards. IEC 60601-1 is a series of technical standards for the safety and effectiveness of medical electrical equipment.
The changes included a revision and renumbering of clauses to align it with the 2005 edition of IEC 60601-1 and other ISOIEC editing requirements. IEC 60601-1 mandates collateral particular and performance standards specific to the device type all of which are required for relevant certification schemes. The IEC 60601-1 Standard itself states ThisStandard applies toMEDICAL ELECTRICAL EQUIPMENT and MEDICAL ELECTRICAL SYSTEMSreferred to as ME EQUIPMENT and ME SYSTEMS.
Amendment to IEC 60601-1 What has A22019 changed compared to A12012. General requirements for basic safety and essential performance INTERNATIONAL ELECTROTECHNICAL COMMISSION CW ICS 11040 PRICE CODE ISBN 978-2-8322-0331-6. Each classification is described in more detail below.
What is IEC 60601-1. IEC 60601-12005 contains requirements concerning basic safety and essential performance that are generally applicable to medical electrical equipment. Your labs ability and background knowledge in medical devices plays.
Standards play a paramount role in product design and development. As planned there is a first Committee Draft for Vote CDV for Amendment 2 toIEC 60601-1This is also referred to as IEC 60601-1 A22019. For certain types of medical electrical equipment these requirements are either supplemented or modified by the special requirements of a collateral or particular standard.
ALL CAPITAL LETTERS identifies a defined term for the IEC 60601 series of standards within this blog. In 2005 the IEC released the 3rd edition which reflected a further change of perspective looking at means of protection MOP both for patients and equipment operators. We have summarised the most important points from the.
General requirements for basic safety and essential performance Collateral standard. Why do I need to classify my product for IEC 60601-1. The main IEC 60601-1 standard referred to in Europe as EN 60601-1 and in Canada as CSA 60601-1 is an umbrella for numerous subsidiary standards variously known as collateral or particular standards.
We are accredited to ISO 17025 are a Certified Body Testing Laboratory CBTL under the IECEE CB Scheme and participate in the UL Data Acceptance Program DAP Intertek Recognized. The main change was in clause 4 where 3rd edition recognizes that IEC 60601-12005 implements a risk management process. Our IEC 60601-1-2 testing clients range from established medical companies to startup entities producing a novel medical device and no single IEC 60601-1-2 testing product or project is the same.
Since IEC 60601-1-8 clearly specifies a measurement radius of 1m it appears to be a technical oversight making the standard impossible to use. General requirements tests and guidance for alarm systems in medical electrical equipment and medical electrical systems. In conjunction with technical documentation and device.
Documentation of ISO 14971 compliant risk management practices must be clear throughout the product lifecycle and device labeling. The 2nd edition published in 1988 focused on safety within the vicinity of a patient. The 4 th edition is strictly one of these collateral standards known as IEC 60601-1-2 Electromagnetic disturbances.
Please contact us with any questions.
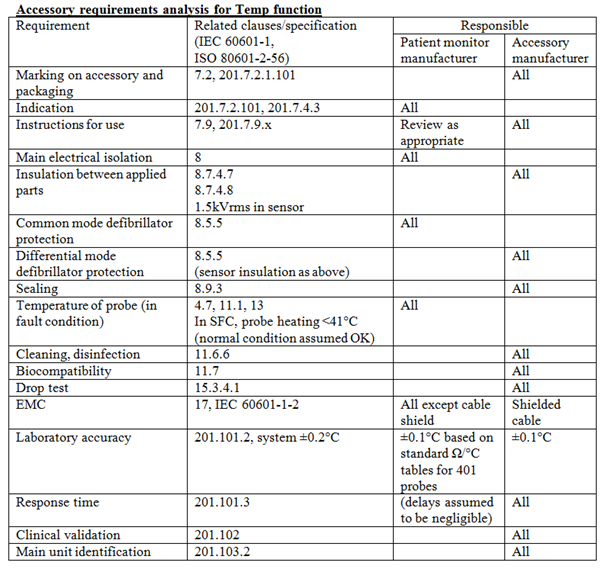
Iec 60601 1 And Accessories Medteq

Benefits Of Using Iec 60601 Compliant Components In Medical Devices
Iec 60601 Medical Design Standards For Power Supplies Plianced Inc

What Is The Iec 60601 Scope Medical Device Academy Medical Device Academy
Iec 60601 Medical Electrical Equipment Classification Faqs Medical Device Academy Medical Device Academy

Iec 60601 1 Amd 1 Ed 3 0 B 2012 Amendment 1 Medical Electrical Equipment Part 1 General Requirements For Basic Safety And Essential Performance

Iec 60601 1 8 2006 Amd2 2020 Amendment 2 Medical Electrical Equipment Part 1 8 General
Medical Emc Iec 60601 1 2 2014 Edition 4 Northwest Emc

International Iec 60601 1 3rd Ed 2nd Amendment An Overview Ris World
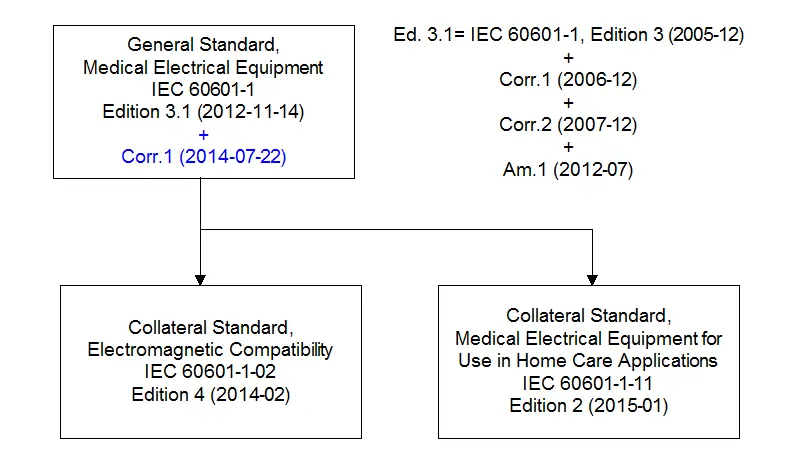
60601 1 Edition 3 1 And Understanding Iec60601 1 Document Structure Globtek
Overview Of Iec 60601 1 Terminology Definitions Through Annexes
Overview Of Iec 60601 1 Terminology Definitions Through Annexes

Iec 60601 1 2 Ed 2 1 B 2005 Medical Electrical Equipment Part 1 2 General Requirements For Safety Collateral Standard Electromagnetic Compatibility Requirements And Tests Iec Tc Sc 62a Amazon Com Books

Iec 60601 1 6 Ed 2 0 B 2006 Medical Electrical Equipment Part 1 6 General Requirements For Basic Safety And Essential Performance Collateral Standard Usability
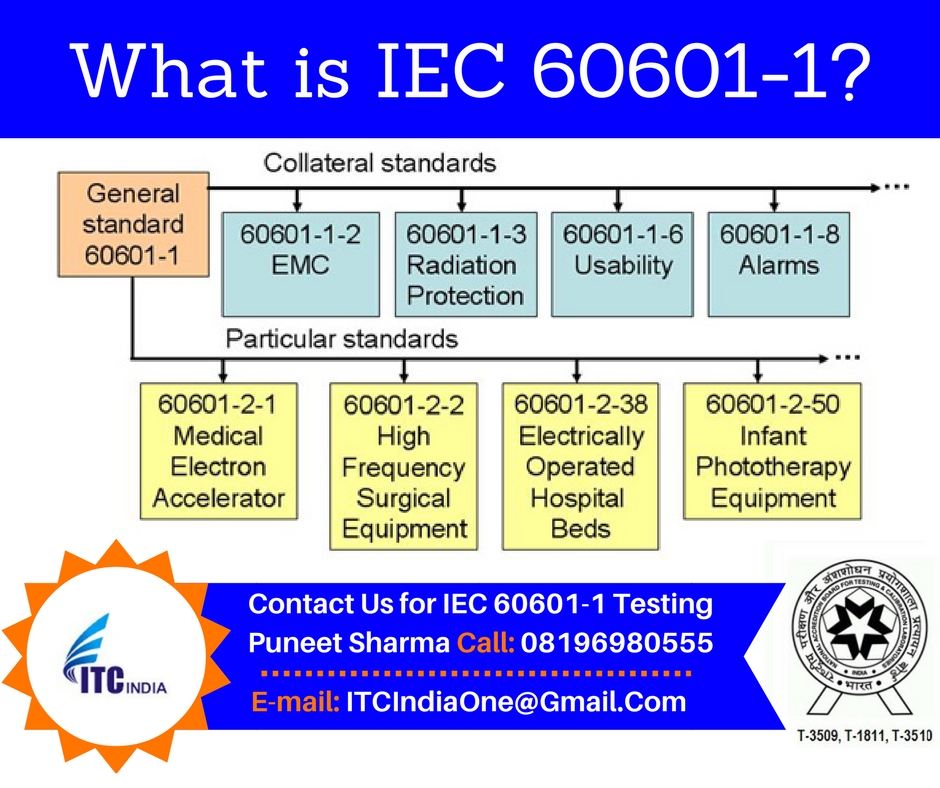
What Is Iec 60601 1 Electrical Safety Testing Lab

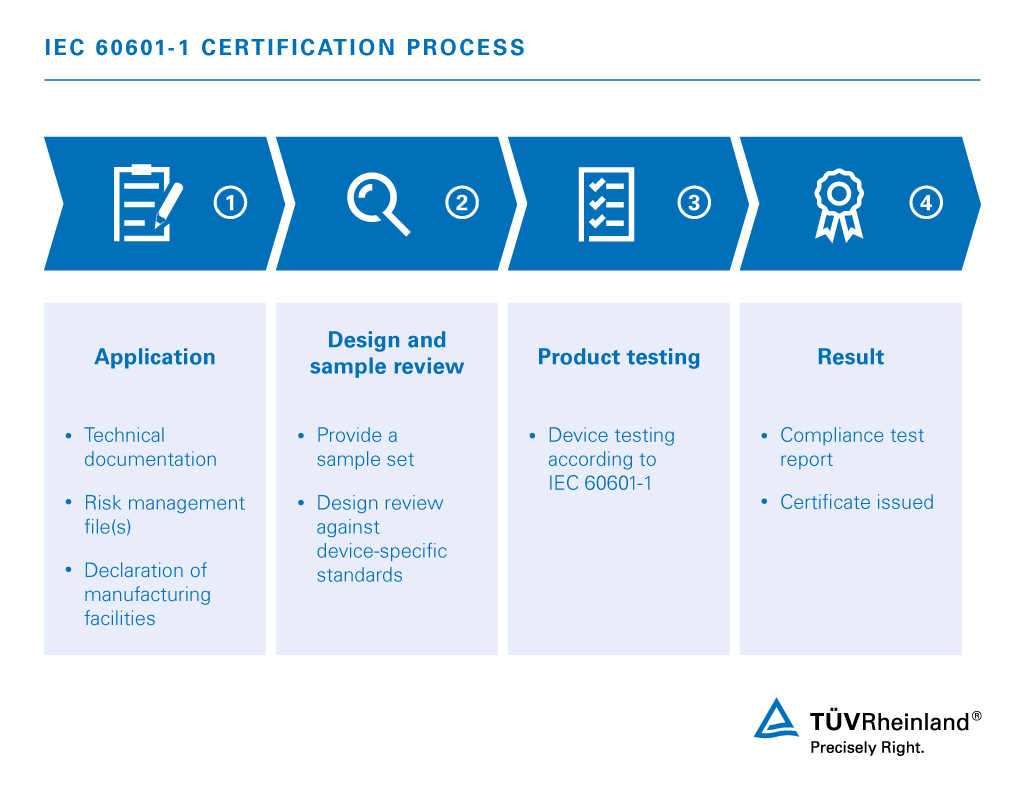
Iec 60601 1 Medical Electrical Equipment Us Tuv Rheinland




Post a Comment
Post a Comment