Iec 62304 / Iec 62304 Medical Device Software Software Life Cycle Processes Verocel
Stay compliant and focus on innovation. ICS 11 11040 1104001.

Implementing Iec 62304 For Safe And Effective Medical Device Software Part 1 Medical Design Briefs
Download iec 62304 Get the latest IEC Standards.
Iec 62304. Advertentie iec 62304 Get the latest IEC Standards. Some minor additional risk management requirements are needed for software especially in the area of identification of contributing software factors related to hazards. IEC 623042006 Medical device software Software life cycle processes.
Buy this standard Abstract Preview. Advertentie Track every change. Company-Wide IEC Codes Subscription Available.
Seamlessly work through compliance requirements and get to market faster. IEC 62304 ISO 13485 Relationship. IEC 623042006Amd 12015 Medical device software Software life cycle processes.
Advertentie iec 62304 Get the latest IEC Standards. The set of processes activities and tasks described in this standard establishes a common framework for medical device software life cycle processes. Download iec 62304 Get the latest IEC Standards.
Defines the life cycle requirements for medical device software. Compliance is critical for medical device developers and there are different requirements based on three IEC 62304 software safety classifications. IEC 62304 is a functional safety standard for medical device software.
Whether its a doctor a specialist or a nurse healthcare providers depend on medical devices to treat their patients. IEC 62304 must be applied in conjunction with ISO 13485 standard which offers a framework for the lifecycle processes necessary for the safe design risk analysis version control and maintenance of standalone software. Buy this standard General information.
Keep all stakeholders informed. The set of PROCESSES ACTIVITIES and TASKS described in this standard establishes a common framework for MEDICAL DEVICE SOFTWARE life cycle PROCESSES. Therefore IEC 62304 makes use of this advantage simply by a normative reference to ISO 14971.
Keep all stakeholders informed. Seamlessly work through compliance requirements and get to market faster. A more recent version of this publication exists IEC 623042006AMD12015 CSV.
Advertentie Track every change. Hét Nederlandse platform voor de opstelling van Europese en internationale normen voor medische elektrische toestellen. Using a tool with an IEC 62304 certification can help speed up.
INTERNATIONAL IEC STANDARD 62304 First edition 2006-05 Medical device software Software life cycle processes This English-language version is derived from the original bilingual publication by leaving out all French-language pages. Missing page numbers correspond to the French-. IECDIS 623043 Health software Software life cycle processes.
Wie hebben er aan deze norm gewerkt. Advertentie Over 6000 IEC Specifications. Those safety-critical systems need to be secure and reliable to ensure everything has been done to prevent any.
As a basic foundation IEC 62304 assumes the guiding principles for the development of and. IEC 623042006 Medical device software - Software life cycle processes. Stay compliant and focus on innovation.
Advertentie Over 6000 IEC Specifications. IEC 62304 is a safety standard for medical devices and compliance with it is critical to software developers. IEC 62304A1 defines the life cycle requirements for MEDICAL DEVICE SOFTWARE.
ICS 11 11040 1104001. Company-Wide IEC Codes Subscription Available.

Achieving Iso 13485 Iec 62304 Medical Device Compliance Tuleap

Setting Up Medical Device Software Development Projects In Compliance With Iec 62304 And Iso 14971 Youtube
Https Www Processvision Nl Templates Processvision Pdf Iec62304 20training Pdf

Iec 62304 2006 En Medical Device Software Software Life Cycle Processes
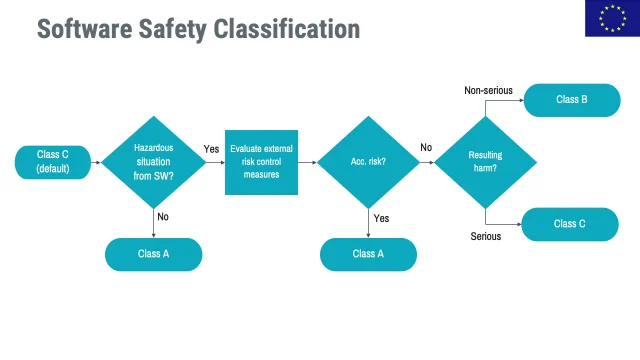
Configuration Management And The Iec 62304 Standard

Relationship Of En 62304 To Other Standards Compliance With The En Download Scientific Diagram

Implementing Iec 62304 For Safe And Effective Medical Device Software Part 2 Ldra

Setting Up Medical Device Software Development Projects In Compliance With Iec 62304 And Iso 14971 In Collaboration With Adesso Ag Intland Software
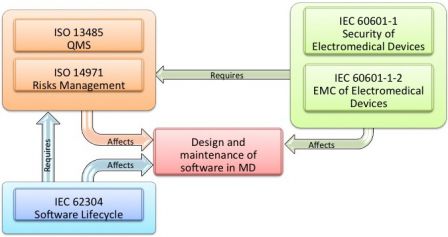
Md And Ivd Standards Iec 60601 1 And Iec 61010 1 Versus Iec 62304 Part 1 Software In Medical Devices By Md101 Consulting

What Is Iec 62304 How Is It Used In Medical Device Compliance

Fda Guidance On Iec 62304 Software Standard Plianced Inc

The Importance Of Iec 62304 Compliance Softcomply

Iec 62304 2006 En Medical Device Software Software Life Cycle Processes

Iec 62304 Standard For Medical Device Software Development Matlab Simulink

Iec Iso 62304 2015 Brightinsight

Iec 62304 2006 Medical Device Software
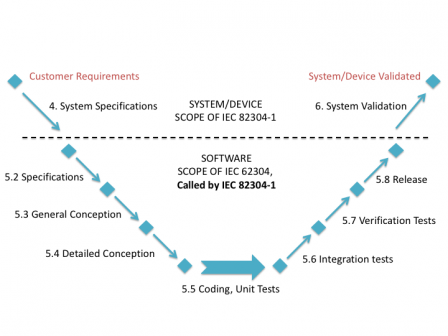
Iec 82304 1 Latest News About The Standard On Health Software Software In Medical Devices By Md101 Consulting

Configuration Management And The Iec 62304 Standard
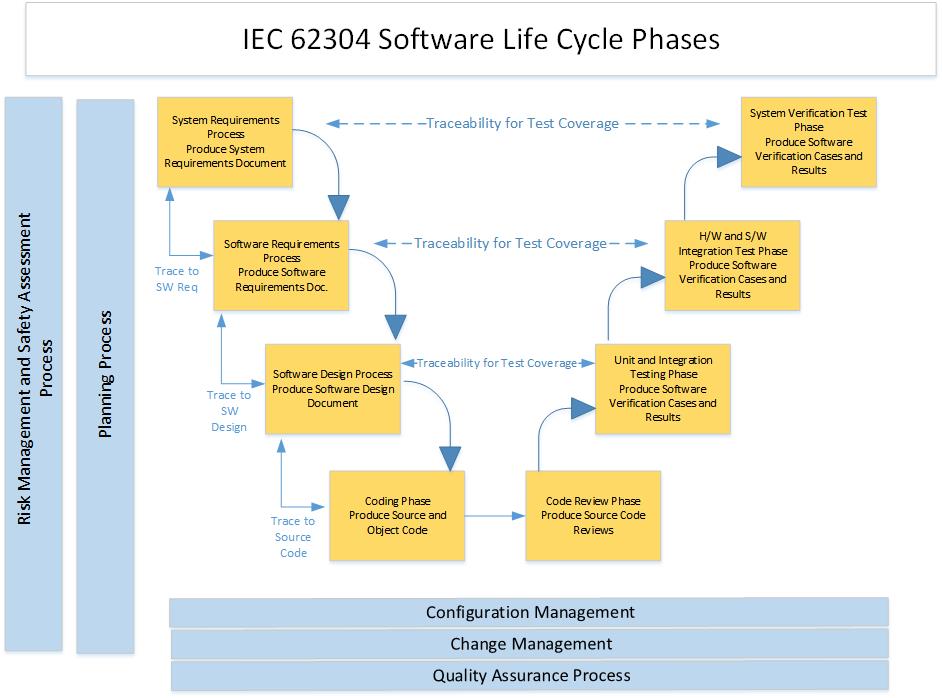
Iec 62304 Medical Device Software Software Life Cycle Processes Verocel

Post a Comment
Post a Comment